The information contained in the site is intended for US healthcare professionals only. By selecting “Okay” below, you verify that you are a licensed US healthcare professional.
By selecting "Okay" below, you verify that you are a licensed US healthcare professional.




Despite a landscape clouded in complexity, emerging biomarkers are expanding our view of patient populations, and biomarker testing could provide a more comprehensive patient profile.


CLDN18.2=Claudin18.2; FGFR2b=fibroblast growth factor receptor 2, isoform IIIb; HER2=human epidermal growth factor receptor 2; MSI=microsatellite instability; PD-L1=programmed death-ligand 1.
AN UNMET NEED
In the United States, approximately 6% of patients with metastatic G/GEJ cancer survive 5 years post diagnosis.1,2*†

*US SEER 22 areas (2013-2019), gastric and esophageal cancers, distant stage.1,2
†SEER data do not have a separate classification for GEJ apart from esophageal cancer; therefore, true GEJ projections are unknown.2
IN THE UNITED STATES
In 2023, an estimated 26,500 new cases of gastric cancer (62% advanced‡ stage) and ~21,600 new cases of esophageal† cancer (72% advanced‡ stage) will be diagnosed in the US.1-3
In the US, patients with advanced disease at diagnosis will likely have a poor outcome,4,5 and less than 50% will receive second-line therapy for mG/GEJ cancer.6§
‡Locally advanced (stage II and III) and metastatic (stage IV) gastric/GEJ cancer per tumor node metastases (TNM) staging classification as described in NCCN Guidelines.4,5
§Data from a retrospective analysis of electronic medical records of 3850 eligible G/GEJ/esophageal adenocarcinoma patients that underwent first-line therapy and were alive at 45 days after completion of first-line therapy.6
TNM=tumor node metastases.
AROUND THE WORLD
Over 1.6 million new cases of gastric and esophagealII cancers were diagnosed worldwide in 2020, making them the 5th and 7th most diagnosed cancers, respectively.7
An estimated 1.3 million people died worldwide in 2020 due to gastric and esophagealII cancers, making them the 4th and 6th most deadly
cancers, respectively.7
IIBased on GLOBOCAN 2020 data for gastric and esophageal cancers. GLOBOCAN data do not have a separate classification for GEJ apart from esophageal cancer; therefore, true GEJ projections are unknown.7
EMERGING BIOMARKERS
help identify previously undefined subsets of patients:
ESTABLISHED BIOMARKERS
are used to inform clinical decisions:
CLDN18.2=Claudin18.2; FGFR2b=fibroblast growth factor receptor 2, isoform IIIb; HER2=human epidermal growth factor receptor 2; MMR=mismatch repair; MSI=microsatellite instability; PD-L1=programmed death-ligand 1.
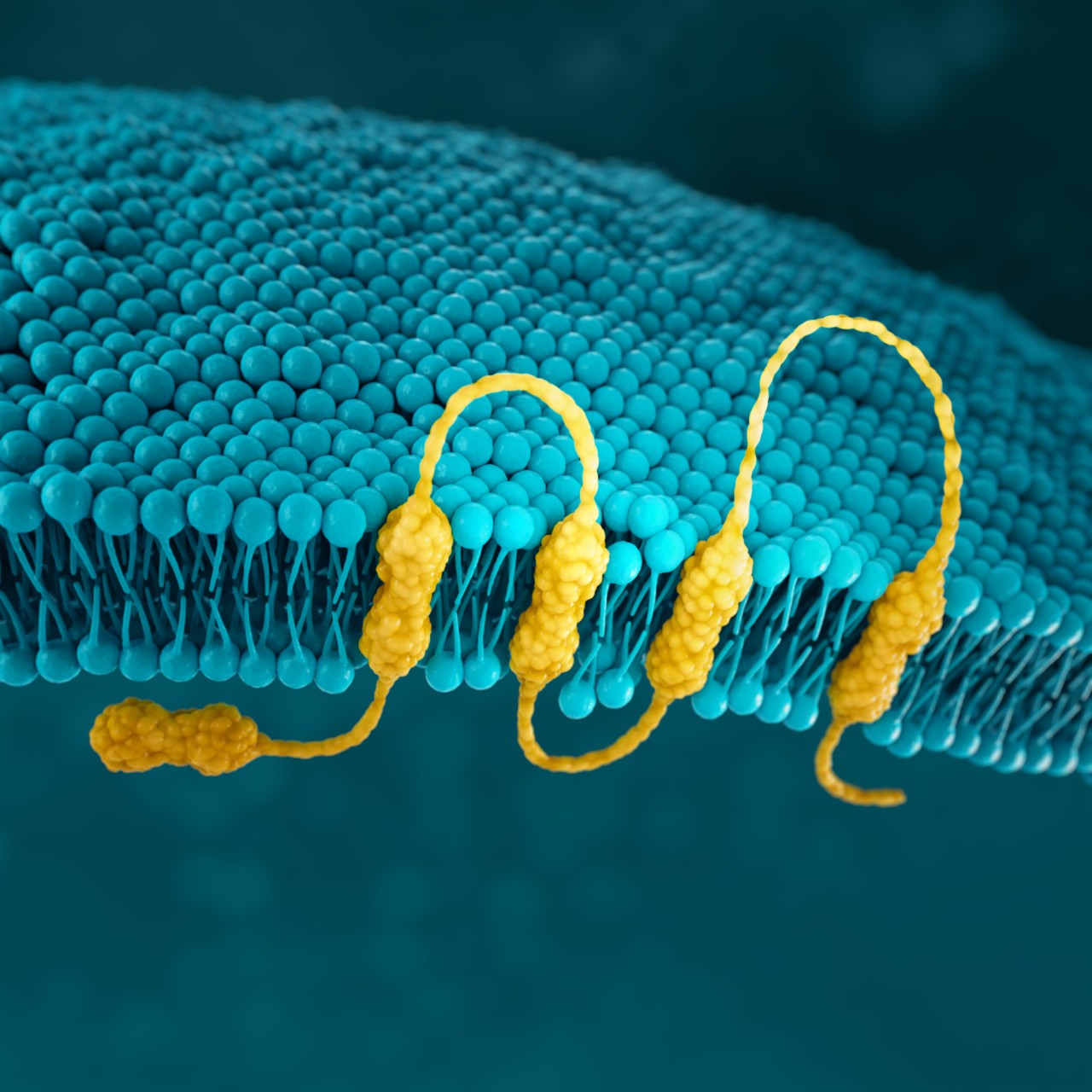
CLDN18.2
Claudins are a family of transmembrane proteins8:
Claudins are a major component of tight junctions, which are involved in controlling the flow of molecules between cells.8,9
Claudins are present throughout the body, but two specific isoforms of CLDN18 are localized to certain tissue types8,17:
Preclinical data have shown that CLDN18.2 may become more exposed
as gastric tumors develop.8,16
CONFINED IN HEALTHY TISSUE
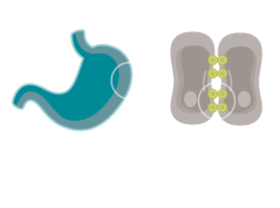

In normal gastric mucosa, CLDN18.2 is typically buried within tight junctions8,16
RETAINED AND EXPOSED IN MALIGNANT TRANSFORMATION
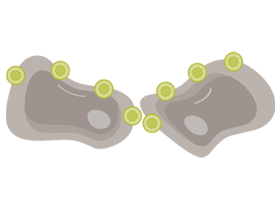

CLDN18.2 is often retained during malignant transformation.
CLDN18.2 may be more exposed when cell polarity disruptions and structure loss occur.8,16,18
MAINTAINED IN METASTATIC PROGRESSION
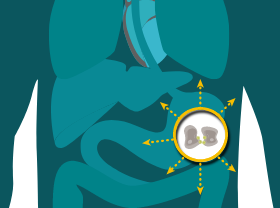

CLDN18.2 may also be localized in lymph node metastases of gastric adenocarcinoma as well as other distant metastatic sites.8,19-21
The information provided above is based on the current understanding of data.
CLDN18.2 expression may also be observed in esophageal adenocarcinoma, pancreatic adenocarcinoma, non-small cell lung cancer, and ovarian mucinous adenocarcinoma.8
While approximately 70% of advanced G/GEJ cancers express CLDN18.2 (at any detectable amount),20* two recent studies have shown that approximately 38% of patients with locally advanced unresectable or mG/GEJ cancer are Claudin18.2 positive (≥75% of tumor cells demonstrating moderate to strong membranous CLDN18 staining by IHC).22,23†
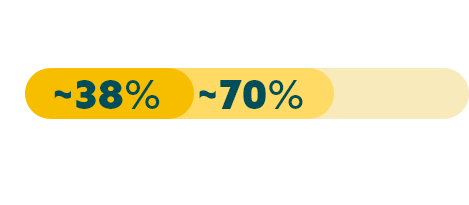

*Data from a retrospective analysis of 350 Caucasian patients with advanced G/GEJ cancer.20
†Data from 2 global randomized Phase 3 studies: the first study included 2,403 assessable patients, of which 922 were CLDN18.2 positive; and a second study which included 2,104 assessable patients, of which 808 were CLDN18.2 positive.22,23
‡Any detectable amount: moderate to strong membranous CLDN18 staining by immunohistochemistry (IHC) in any percentage of tumor cells.20
Biomarker prevalence estimates from select studies are reported below. Prevalence data can vary among studies due to tumor heterogeneity, differences in patient population, clinical trial methodology, and diagnostic assays used. Positivity thresholds also vary by study.13,22-24,26-28
EMERGING BIOMARKERS
CLDN18.222,23
(positive)§
38%
FGFR2b26||
(positive)
30%
§≥75% of tumor cells demonstrating moderate to strong membranous CLDN18 staining by IHC.22,23
||FGFR2b positivity: FGFR2b overexpression (IHC 2+/3+ any amount of tumor cells) and/or FGFR2 gene amplification by ctDNA (NGS 1.5x increase in FGFR2).26
ESTABLISHED BIOMARKERS
PD-L124,27
(variable due to multiple factors)¶
CPS ≥1: 67-73%
CPS ≥5: 29-31%
CPS ≥10: 16-18%
HER213
(positive)
22%
MSI28
(MSI-high)
4%
CPS=combined positive score.
¶ PD-L1 prevalence at various CPS thresholds is still being explored. Data are from a randomized controlled trial and a real-world retrospective medical records study.24,27
EMERGING BIOMARKERS
CLDN18.2:
IHC22,23
FGFR2b:
IHC, NGS (ctDNA)26#
#FGFR2b protein overexpression assessed by IHC; FGFR2 gene amplification assessed using ctDNA by NGS.26
ESTABLISHED BIOMARKERS
PD-L1:
IHC4,5**
HER2:
IHC, ISH, NGS4,5,25††
MMR/MSI:
IHC, PCR/NGS4,5‡‡
ctDNA=circulating tumor DNA; IHC=immunohistochemistry; ISH=in situ hybridization; NGS=next generation sequencing; PCR=polymerase chain reaction.
** Varying diagnostic assays.29
†† Other ISH methods (FISH=fluorescent ISH; SISH=silver ISH; CISH=chromogenic ISH; DDISH=dual-color dual-hapten ISH).25
‡‡ MMR assessed by IHC, MSI by PCR/NGS.4,5
IHC scoring for CLDN18 in G/GEJ cancer is performed based on membrane staining intensity and percent of positive tumor cells22,23,30
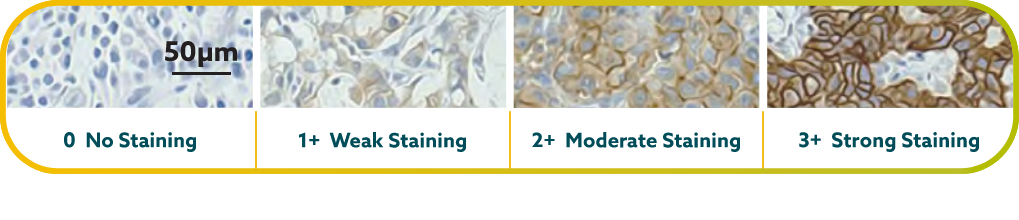
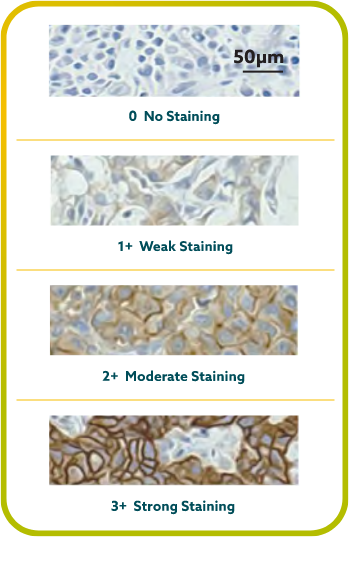
CLDN18.2 positivity is defined as ≥75% of tumor cells demonstrating moderate to strong membranous CLDN18 staining by IHC.22,23
CLDN18.2 expression has been seen in both diffuse-type gastric tumors and intestinal-type gastric tumors.20
FGFR2b
FGFR2b participates in angiogenesis and cell proliferation through
FGFR signaling pathways.11,12
FGFR2b positivity has been observed in 30% of advanced G/GEJ cancers.26*


FGFR2b positivity: FGFR2b overexpression (IHC 2+/3+ any amount of tumor cells) and/or FGFR2 gene amplification by ctDNA (NGS 1.5x increase in FGFR2)
*Data from select studies.26
Detecting FGFR2b can be done with the following tests26:
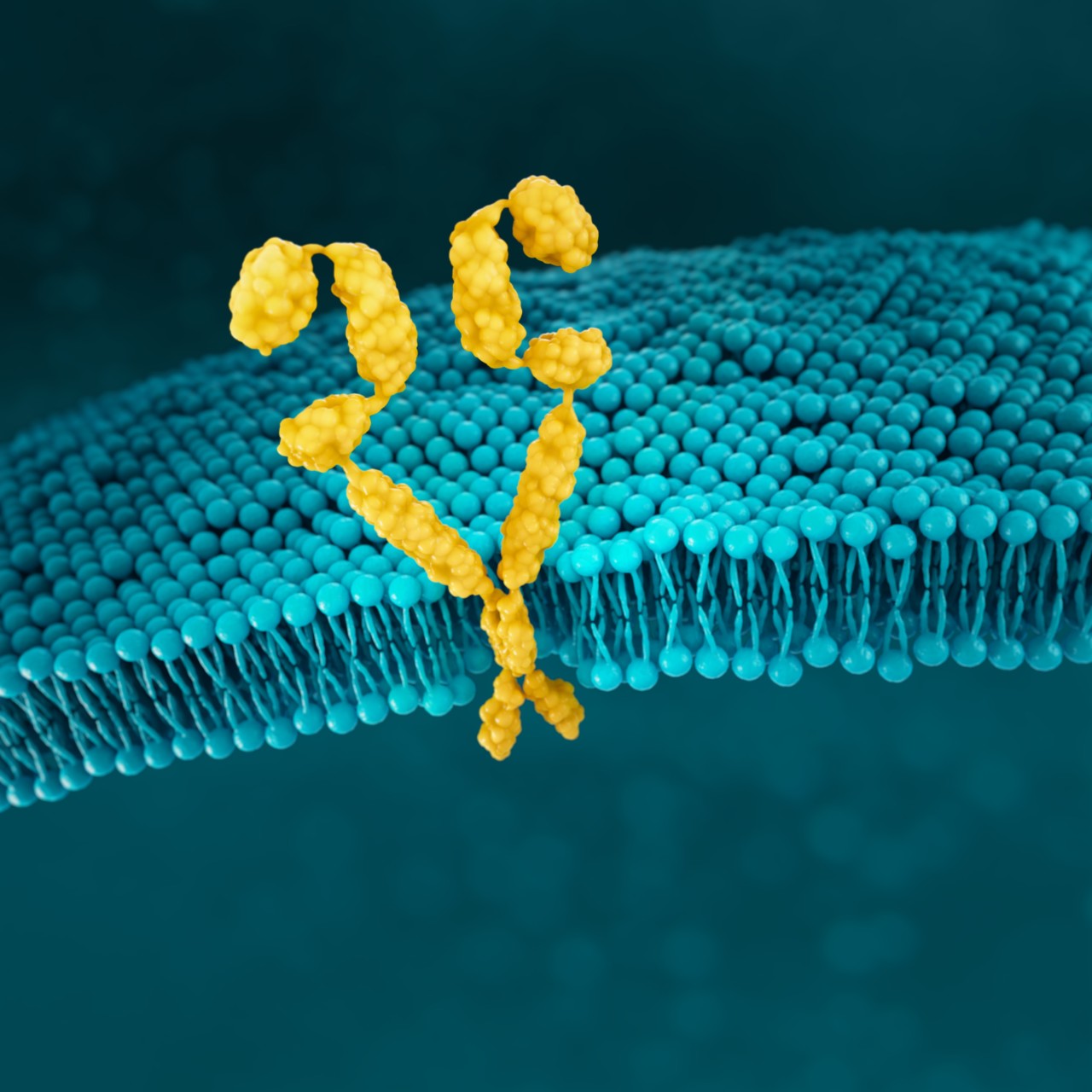
HER2
HER2 (human epidermal growth factor receptor 2) is a receptor-tyrosine kinase that is overexpressed and/or amplified in advanced G/GEJ cancer.11
HER2 is a proto-oncogene that is involved in signaling pathways, which leads to cell growth and differentiation.25
HER2 positivity has been reported in 22% of advanced G/GEJ cancers.13*


HER2 positivity: overexpression (IHC3+) and/or gene amplification (FISH-positive)
*Data from select studies.13
Detection of HER2 may be done with IHC, NGS, and ISH methods.4,5
MSI
MSI is associated with genomic instability and increased susceptibility to tumor development.11
Microsatellites are repeated sequences of nucleotides in DNA.14
MSI-H has been reported in 4% of advanced G/GEJ cancers.28*


*Data from select studies.28
Detection of MSI and MMR is typically assessed with various methods.4,5
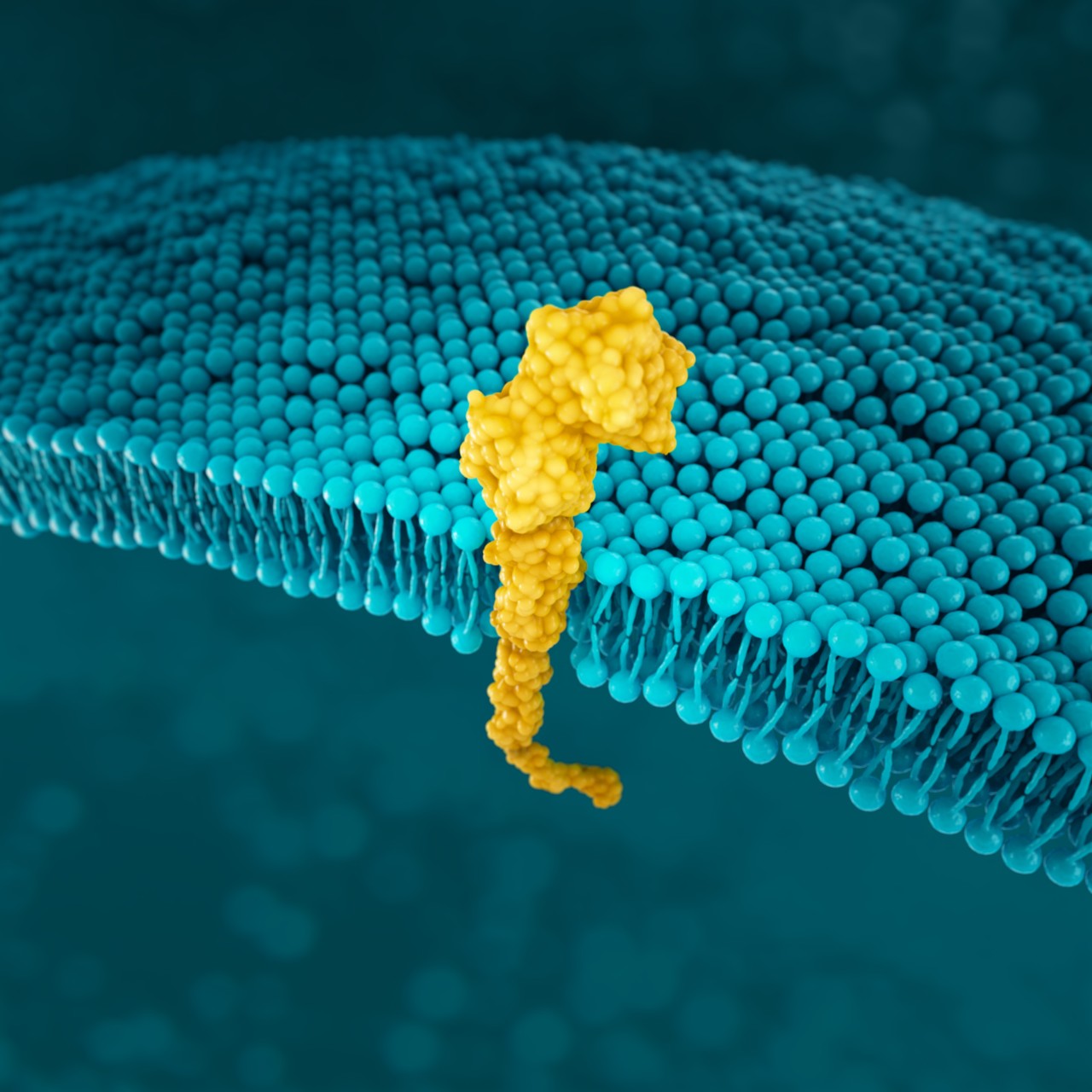
PD-L1
PD-L1 (programmed death-ligand 1) is a transmembrane protein that may be expressed on various tumor cells and/or immune cells.37
Prevalence of PD-L1 has been reported for several positivity thresholds throughout various studies24,27*:






PD-L1 prevalence at various CPS thresholds is still being explored. Data are from a randomized controlled trial and a real-world retrospective medical records study.24,27
*Data from select studies.24,27
PD-L1 expression is detected using IHC.4,5
CPS=combined positive score.
SUMMARY
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastric Cancer and Esophageal and Esophagogastric Junction Cancers* support using biomarkers to help map the path forward for patients.4,5†
The NCCN Guidelines® recommend4,5:
*In adenocarcinomas of unresectable locally advanced, locally recurrent or metastatic disease of esophageal and esophagogastric junction cancers.5
†This is a summary of relevant portions of the NCCN Guidelines. Please see the full NCCN Guidelines for Gastric Cancer and Esophageal and Esophagogastric Junction Cancers at NCCN.org.4,5
Biomarker testing provides more insight into advanced G/GEJ cancer as more biomarkers are discovered.
‡The use of IHC/ISH/targeted PCR should be considered first, followed by additional NGS testing as appropriate.4,5
§Data from select studies. Prevalence data can vary among studies due to tumor heterogeneity, differences in patient populations, clinical trial methodology, and diagnostic assays used. Positivity thresholds may vary by study.13,24,26-28
As biomarker research continues, it expands our view of the patient population, reveals more information about the advanced G/GEJ cancer landscape, and helps inform clinical decisions.

Have a question?
Find answers at
astellasanswers.com.
Stay up to date
By signing up below, you will receive updates about emerging biomarkers in advanced G/GEJ cancer. You may also be contacted by an Astellas representative. Please note that fields marked with (*) are required.
You will now receive email updates about emerging biomarkers in mG/GEJ cancer, and you may be contacted by an Astellas representative.
REFERENCES
The information provided on gastriccancerbiomarkers.com is intended for residents of the United States.
© 2023 Astellas Pharma US, Inc. All rights reserved.
WEBSITE TERMS OF USE (Effective date: 4/22)
ATTENTION: PLEASE READ THESE TERMS CAREFULLY BEFORE USING THIS WEBSITE GASTRICANCERBIOMARKERS.COM. BY USING THIS SITE, INCLUDING ANY SERVICES PROVIDED ON OR THROUGH THIS SITE GASTRICANCERBIOMARKERS.COM, YOU INDICATE THAT YOU ACCEPT THESE TERMS. IF YOU DO NOT ACCEPT THESE TERMS, DO NOT USE THIS SITE.
Astellas Pharma US, Inc., (Astellas) may at any time revise these Terms of Use by updating this posting, so you should periodically examine the current Terms of Use to which you are bound. Certain provisions of these Terms of Use may be superseded by expressly designated legal notices or terms located on particular pages at this Site. To view the Terms of Use at any time, go to GastricCancerBiomarkers.com.
GENERAL INFORMATION. This Site contains information that may be of interest to members of the healthcare community. Please feel free to browse this Site and Services. Your access and use of the Site are subject to the following terms and conditions, and all applicable laws. By accessing and browsing this Site and Services, you accept, without limitation or qualification, these terms and conditions and acknowledge that they supersede any other agreement between you and Astellas.
PRIVACY. Astellas's policies concerning the use of your personal information are set forth in Astellas's Privacy Policy, which you can find by clicking here, and which are incorporated by reference herein. By using this Site or any services provided by or through this Site (Services), you agree to waive and release Astellas from any claim or liability in connection with the collection, use, or disclosure of information consistent with Astellas's Privacy Policy.
SITE CONTENT. All material on the Site including, but not limited to, text, images, graphics, documents, audio, or video (collectively, the Site Content) are protected under applicable copyright laws. You may copy, download, or print Site Content for your personal, noncommercial purposes only, but no modification or further reproduction of content is permitted. The Site Content may not otherwise be copied, downloaded, reproduced, deleted, modified, republished, retransmitted, displayed, or used to create derivative works without the express written permission of Astellas.
Trademarks, service marks, trade dress, logos, designs, and slogans appearing on this Site are owned by Astellas, its Affiliate companies, its licensors, or its partners, and may not be used for advertising or publicity purposes, or to indicate any affiliation with or endorsement by Astellas, except with the express written permission of Astellas.
Nothing contained in this Site should be construed as granting a license to, or right in, any trademarks, patents, or copyrights of Astellas, or any other party.
You are solely responsible for any content, including but not limited to text, photographs, caricatures, illustrations, designs, icons, articles, audio clips, and video clips (collectively, User Content) that you post, email, or otherwise transmit via this Site. Astellas does not own or endorse User Content posted or otherwise transmitted via this Site, and Astellas does not guarantee the accuracy, integrity, or quality of such User Content. Any communications you send via this Site or otherwise to Astellas by email, with the exception of personally identifiable information as defined in Astellas's Privacy Policy, shall be deemed to be nonconfidential and Astellas shall have no obligation of any kind with respect to such information and shall be free to reproduce, use, disclose, and distribute the information to others without limitation. Astellas shall be free to use any ideas, concepts, know-how, or techniques contained in such information for any purpose whatsoever, including but not limited to developing, manufacturing, and marketing activities incorporating such information.
The content or material Astellas provides through the Site is for informational purposes only. Nothing on this Site should be construed as the giving of advice (medical, legal, financial, investment, or other professional advice) or the making of a recommendation, and information on the Site should not be relied on as the basis for any decision or action. IT IS IMPORTANT THAT YOU RELY ONLY ON THE ADVICE OF A HEALTHCARE PROFESSIONAL TO ADVISE YOU ON YOUR SPECIFIC SITUATION. YOU SHOULD NEVER DISREGARD OR DELAY SEEKING MEDICAL ADVICE BECAUSE OF SOMETHING THAT YOU HAVE SEEN ON THIS SITE.
OBLIGATIONS OF SITE VISITORS OR USERS. You agree not to do any of the following while visiting or using the Site or any services provided by or through this Site:
Astellas may, in its sole discretion, take any action it deems necessary, including but not limited to termination of access to the Site or modification of User Content, if Astellas believes a user's conduct or content fails to conform to these Terms of Use or may create liability for Astellas, its partners, its suppliers, or any other party or person. Astellas will not be liable to you or any third party as a result of such termination or modification. The terms and conditions provided in these Terms of Use will survive any such termination and modification.
LINKS TO OTHER WEBSITES. Solely as a convenience to you, Astellas may provide links on this Site to other websites that are not maintained by or under the control of Astellas. If you use these links, you will leave this Site. Please remember that linked sites are not necessarily Astellas's sites, and the information could change without Astellas's knowledge. Third-party linked sites and their contents should not be attributed to Astellas (or any of its affiliated companies) in any manner. Astellas has not attempted to verify the truth or accuracy of any third-party sites or content contained on any third-party sites and does not make any representations or warranties about such sites or the content of such sites. If you decide to access any of the third-party sites linked to this Site, you do so entirely at your own risk. In the event Astellas endorses a particular organization, Astellas will clearly state its endorsement next to any link to that organization.
DISCLAIMER OF WARRANTIES. THIS SITE, INCLUDING ANY INFORMATION, SERVICES, OR MATERIAL MADE AVAILABLE ON OR THROUGH THIS SITE, IS PROVIDED ON AN "AS IS" AND "AS AVAILABLE" BASIS. TO THE MAXIMUM EXTENT PERMITTED BY LAW, ASTELLAS EXPRESSLY DISCLAIMS ALL WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING WITHOUT LIMITATION ANY WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR NONINFRINGEMENT. FURTHERMORE, ASTELLAS DOES NOT WARRANT THE ACCURACY OR COMPLETENESS OF ANY INFORMATION ON THIS SITE AND MAY MAKE CHANGES TO THE INFORMATION, SERVICES, OR MATERIAL MADE AVAILABLE ON OR THROUGH THIS SITE AT ANY TIME WITHOUT PRIOR NOTICE TO USERS OF THIS SITE. INFORMATION AT THIS SITE THAT IS PERIODICALLY UPDATED MAY NOT BE CURRENT AT THE MOMENT YOU VISIT THIS SITE AND MAY CONTAIN ERRORS. ASTELLAS MAKES NO WARRANTY THAT THE SITE WILL MEET YOUR REQUIREMENTS; THAT THE SITE WILL BE UNINTERRUPTED, TIMELY, SECURE, OR ERROR-FREE; THAT MESSAGES OR REQUESTS WILL BE DELIVERED; THAT DEFECTS WILL BE CORRECTED; OR THAT THIS SITE IS FREE OF VIRUSES OR OTHER HARMFUL COMPONENTS. ASTELLAS MAKES NO WARRANTY REGARDING ANY GOODS OR SERVICES PURCHASED OR OBTAINED THROUGH THE SITE OR ANY TRANSACTIONS ENTERED INTO THROUGH THE SITE. YOU ASSUME THE ENTIRE RISK AS TO THE RESULTS AND PERFORMANCE OF THE SITE AND ANY GOODS OR SERVICES PURCHASED THEREFROM.
LIMITATION OF LIABILITY. IN NO EVENT WILL ASTELLAS BE LIABLE FOR ANY DAMAGES WHATSOEVER. THIS INCLUDES BUT IS NOT LIMITED TO DIRECT, INDIRECT, INCIDENTAL, PUNITIVE, AND CONSEQUENTIAL DAMAGES (INCLUDING, WITHOUT LIMITATION, THOSE RESULTING FROM LOST PROFITS, LOST DATA, OR BUSINESS INTERRUPTION) ARISING OUT OF THE USE, INABILITY TO USE, OR THE RESULT OF USE OF THIS SITE, ANY WEBSITES LINKED TO THIS SITE, THE MATERIALS OR INFORMATION CONTAINED ON ANY OR ALL SUCH SITES, OR ANY MATERIALS, PRODUCTS, OR SERVICES OFFERED ON THIS SITE OR SITES LINKED TO THIS SITE, WHETHER BASED ON WARRANTY, CONTRACT, TORT, OR ANY OTHER LEGAL THEORY AND WHETHER OR NOT ADVISED ON THE POSSIBILITY OF SUCH DAMAGES. IN NO EVENT WILL ASTELLAS, ITS SUPPLIERS, OR ANY OTHER PARTY INVOLVED IN CREATING, PRODUCING, OR DELIVERING THIS SITE BE LIABLE TO YOU IN ANY MANNER WHATSOEVER FOR ANY DECISION MADE OR ACTION OR NONACTION TAKEN BY YOU IN RELIANCE UPON INFORMATION PROVIDED THROUGH THIS SITE. APPLICABLE LAW MAY NOT ALLOW THE EXCLUSION OR LIMITATION OF INCIDENTAL OR CONSEQUENTIAL DAMAGES, SO THE ABOVE LIMITATION OR EXCLUSION MAY NOT APPLY TO YOU.
THIS SITE IS INTENDED FOR US AUDIENCES ONLY. Access to and use of this Site is subject to the above terms and conditions. This Site was developed and is maintained by Astellas. Information, copy, and claims are intended only for the residents of the United States.